Cyto Conference 2024
Edinburgh International Convention Center 150 Morrison St, Edinburgh, United KingdomJoin Discovery Life Sciences at Cyto Conference 2024 May 4-8, 2024 | Edinburgh International Convention Center, Edinburgh, Scotland
Cytochrome P450 and UGT induction studies to predict drug-drug interaction.
We have 20+ years of expertise in regulatory compliant ADME services. We provide in-process communication, IND submission-ready reports, and reliable results using Gentest® products and services.

Robust Dynamic Range to Assess Induction Response
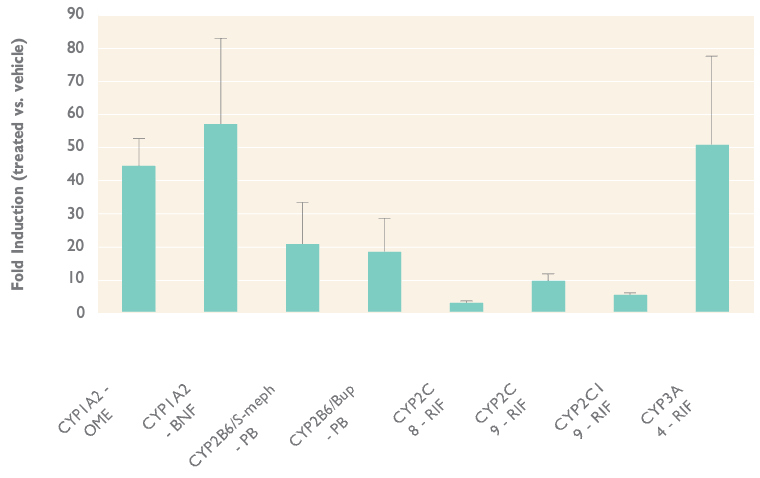
Flexibility to use multiple inducers or assays for the same enzyme with robust analytical sensitivity. Mean and standard deviation of fold induction of enzyme activity in 3 donors after treatment of 50 μM omeprazole (OME), 20 μM ß-naphthoflavone (BNF), 1 mM phenobarbital (PB), or 10 μM rifampicin (RIF). Enzyme activities measured were phenacetin O-deethylase, S-mephenytoin N-demethylase, bupropion hydroxylase, amodiaquine N-deethylase, diclofenac 4’-hydroxylase, S-mephenytoin 4’-hydroxylase, and testosterone 6ß-hydroxylase.
Reproducible Induction Response in Gentest® Inducible-qualified Human Cryo-hepatocytes
Omeprazole-induced CYP1A2 catalytic activity in Gentest® Inducible-qualified human cryo-hepatocytes on 7 independent days
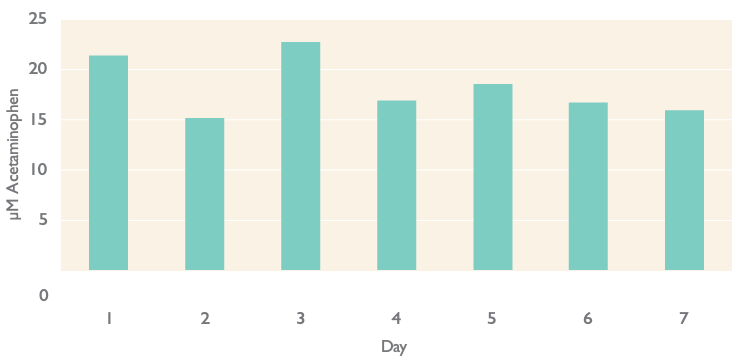
Highly reproducible, robust data. Phenacetin O-deethylase activity in a single donor (lot 246) of Gentest® Inducible-qualified human cryopreserved hepatocytes in 7 independent experiments conducted over the span of 18 weeks. Cells were treated with 50 μM omeprazole (OME) for 2 days prior to determination of enzyme activity. The amount of acet- aminophen metabolite formation was determined by incubating cells with 100 μM phenacetin for 30 minutes.
Zhang JG, et al (2014). Evaluation of calibration curve-based approaches to predict clinical inducers and non-inducers of CYP3A4 with plated human hepatocytes. Drug Metab. Dispos. 42:1379-1391.
Wong, S.G. et al (2021). Considerations from the innovation and quality induction working group in response to drug-drug interaction guidance from regulatory agencies: Guidelines on model fitting and recommendations on time course for in vitro cytochrome P450 induction studies including impact on drug interaction risk assessment. Drug Metab. Dispos. 49:94-110.

Join Discovery Life Sciences at Cyto Conference 2024 May 4-8, 2024 | Edinburgh International Convention Center, Edinburgh, Scotland
Join Discovery Life Sciences at APT 2024 May 14-16, 2024 | Merck Research Laboratories, Boston, Massachusetts
Join Discovery Life Sciences at ISCT 2024 Stop by our booth #453 or schedule a meeting with our CGT team. May 28- 31, 2024 | Vancouver, Canada
Copyright © 2024 Discovery Life Sciences. All rights reserved.
Designed & Developed by Altitude Marketing