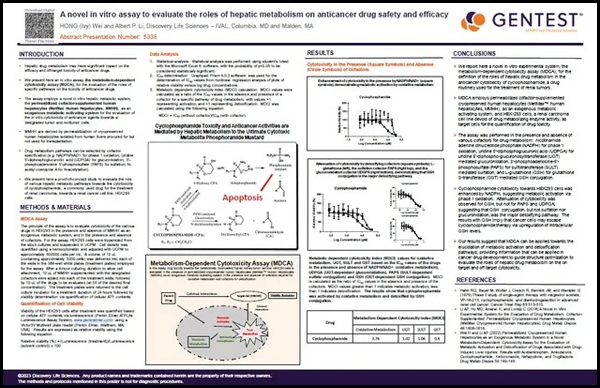
Hepatic drug metabolism may have significant impact on the efficacy and off-target toxicity of anticancer drugs. We present here an in vitro assay, the metabolism-dependent cytotoxicity assay (MDCA), for the evaluation of the roles of specific pathways on the toxicity of anticancer drugs.
The assay employs a novel in vitro hepatic metabolic system, the permeabilized cofactor-supplemented human hepatocytes (MetMax Human Hepatocytes, MMHH), as an exogenous metabolic activating system for the evaluation of the in vitro cytotoxicity of anticancer agents towards a designated tumor and nontumor cells.
MMHH are derived by permeabilization of cryopreserved human hepatocytes isolated from human livers procured for but not used for transplantation. Drug metabolism pathways can be selected by cofactor specification (e.g. NADPH/NAD+ for phase 1 oxidation; Uridine 5′-diphosphoglucuronic acid (UDPGA) for glucuronidation; 3′-phosphoadenosine 5′-phosphosulfate (PAPS) for sulfation; N-acetyl coenzyme A for N-acetylation).
We present here a proof-of-concept study to evaluate the role of various hepatic metabolic pathways towards the cytotoxicity of cyclophosphamide, a commonly used drug for the treatment of renal carcinoma, towards a renal cancer cell line, HEK293 cells.