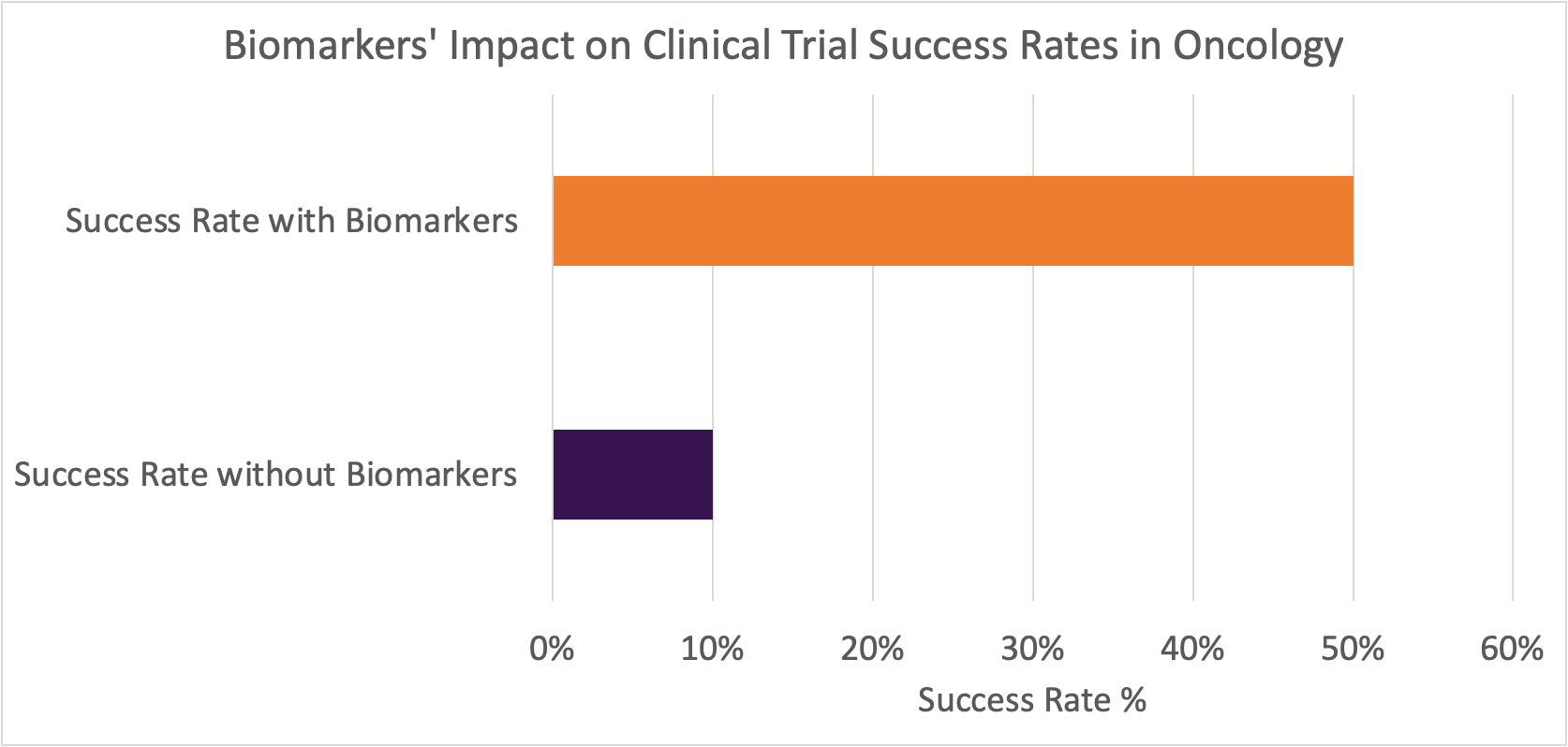
According to a recent peer-reviewed paper published in the journal of Cancer Medicine, the clinical trial failure rates for drugs treating major cancer types (non-small cell lung cancer, metastatic melanoma, non-Hodgkin’s lymphoma, and prostate cancer) averages 90%. The significance of this high failure rate and its impact on global health is amplified when considering the prevalence rates and the number of newly launched clinical trials associated with these cancer types. As a consequence, billions of dollars are wasted and millions of patients are negatively affected by this grim reality. However, biomarkers hold great potential to curb these failure rates as illustrated by the extensive analysis performed by the authors of the Cancer Medicine journal who concluded that the inclusion of biomarkers during drug development increases the likelihood of approval fivefold.
At Discovery, our scientists intricately understand the role biomarkers play in the success of precision oncology programs and are dedicated to designing biomarker strategies that help researchers discover, develop, and validate novel biomarker assays more quickly and cost-effectively. To do so, we employ optimized processes, consistent scientific guidance, and prioritize long term considerations like our clients’ projected timelines, goals, and resources.
What we often encounter is that researchers recognize the importance of biomarkers, but do not always know how to streamline their use to efficiently navigate precision oncology programs. We provide consultative services that tailor assays for the right targets in the right patients on the right platforms. Ultimately, this saves our clients both time and money while helping them to improve patient care for millions worldwide.
Dr. Tiffany Murphy
VP of Discovery’s Global Tissue Biomarker Services
Whether a biomarker is used in exploratory phases, as inclusion/exclusion criteria in clinical trials, or is developed as a diagnostic assay, developers need to have a solid, well-thought out biomarker strategy to be successful. With over 100 years of combined experience, our biomarker experts serve as a common thread throughout development to deliver the data scientists need to overcome common challenges.
Top 3 challenges when using biomarkers in precision oncology programs
Challenge 1: Understanding Expression Patterns to Navigate Go/No-Go Decisions
Many late-stage clinical trials fail due to inadequately distinguishing between disease phenotypes and underlying drivers of disease early on in development. This impacts their biomarker targets’ ability to accurately predict disease, prognosis, and therapeutic response. In addition to predictive biomarkers, our customers may also need to use separate pharmacodynamic (PD) markers as well. Researchers must adequately understand expression patterns and base decisions made during early development on data derived from high-quality study cohorts that are diverse, comprehensive, highly characterized and representative of the target population.
Discovery’s Tissue Biomarker Services division provides data to gain this understanding by conducting large-scale sensitivity screening of cancer patient populations and controls using IHC assays that can be run on Dako, Leica, TechMate and Ventana platforms. The data generated is then used to capture the level of target expression in various tumor indications, allowing for prioritization of indications with the highest expression that are most likely to respond to the targeted therapy. We can also confirm results across sample matrices using matched biospecimen sets and multi-omic analyses (flow cytometric and genomic) to ensure robust results.
However, these evaluations are only as good as the quality of the biospecimen cohorts used, so we provide access to our biospecimen inventory, the largest commercial biorepository of biospecimens, that are highly characterized with a single chain of custody and rigorous, pathologist-guided quality control assessments.
“Discovery’s scientists ensure that a comprehensive analysis of expression patterns is conducted, highlighting cell and tissue type expression likely to lead to a higher probability of therapeutic benefit,” said Dr. Greg Cesarone, VP of Discovery’s Global Tissue Biomarker Services. “We are uniquely positioned to help clients do this given our platform agnostic, multi-omic approach and unparalleled access to highly annotated, quality biospecimens.”
Challenge 2. Accurately Validating Assays
The parameters required for a ‘fit for purpose’ clinical validation, in preparation for patient selection, include sensitivity, specificity, and reproducibility inclusive of testing around selected cutoff points – ultimately used as part of the process for screening patients in a clinical trial. Epitope and analyte stability studies are optional but provide greater confidence in the use of the test.
Discovery demonstrates precision and reproducibility using serial sections from samples with various levels of known target expression from the large-scale sensitivity screening described in Challenge 1. We analyze specificity in cell lines, xenografts, and normal human tissues – e.g. tissue cross reactivity (TCR) analysis typically via a tissue microarray (TMA).
Typically, we select four samples for our precision and reproducibility to test with known target expression from high, moderate, negative, and low expression. These four samples are tested in three separate runs by different operators in triplicate on different runs (nine replicates). See the P & R image below.

Precision and reproducibility testing is formatted such that samples from each expression level (high,moderate, low and negative) are tested in 3 different runs (only high shown here), by at least 2 different operators, in triplicate. In each run, an isotype control (e.g. Rb IgG,Ms IgG1, Ms IgG2a depending on primary antibody) is also tested as the negative control (bottom row).
Discovery has validated a broad menu of multiplex and single-plex assays for human and mouse immuno-oncology markers, here are some examples of single-plex, human specific validated IO assays in our service menu:
- CD3 (Total T-cells)
- CD4 (T-helper cells)
- FOXP3 (T-regs)
- CD20 (B-cells)
- CD57 (NK cells)
- CD68 (macrophages)
- CD163 (macrophages)
- Ki67 (proliferating cells)
- PD-1 (immune checkpoint receptor)
- PD-L1 (PD-1 ligand)
In addition to evaluating immune cells in human tumor tissue, we have also developed a panel of IHC assays to detect the present and distribution of immune cells in mouse tissue as well as various validated assays in place for mouse immune markers.
Challenge 3. Submitting Data to Regulatory Authorities
Submission to regulatory authorities is a major milestone in any precision oncology program. The requirements evolve over time and may differ widely depending on the regulatory body.
Most commonly, our clients are working toward an Investigational Device Exemption (IDE) to use their assay in clinical studies, as well as CE labeling to gain access to global trials
We provide support to our customers with their IDE/CE submission. Our assay development and validation reports save our customers’ time as they are designed to be incorporated, without modification, as part of an IDE/CE submission. We have the experience of testing samples from over 600 clinical trials (prospective and retrospective testing) and developing multiple prototype companion diagnostic tests that can continue to be used in our labs up to and beyond commercialization of these tests thus supporting the Sponsor before widespread availability of their commercial test. Importantly, we can also offer educational training for pathologists as the commercial test becomes available. Lastly, we can validate the prototype test in multiple labs within the US/EU to support Sponsor studies in additional indications beyond first approval as well as carry out testing to support Investigator led studies.
Conclusion:
Discovery helps clients plan strategically throughout development to use biomarkers that increase the likelihood of success in their precision oncology programs. We guide developers from biomarker discovery through assay validation and clinical trial testing. Furthermore, we carefully assess target expression using large-scale screening studies to make sure our clients understand the drivers of disease and their biomarker’s ability to measure them robustly. Discovery conducts sensitivity, specificity, stability and reproducibility assessments for assay validation, provides testing from early phase trials through to global clinical trials, and helps prepare reports for regulatory submissions. Our experts serve as a common thread throughout development and have demonstrated the success of our approach in dozens of peer-reviewed publications and leading companion diagnostic/precision oncology programs such as in the cases of HER2 and 22C3 PD-L1.
Read more about how we’ve helped advance leading precision oncology programs: https://www.dls.com/her2-and-pdl1-case-studies/


