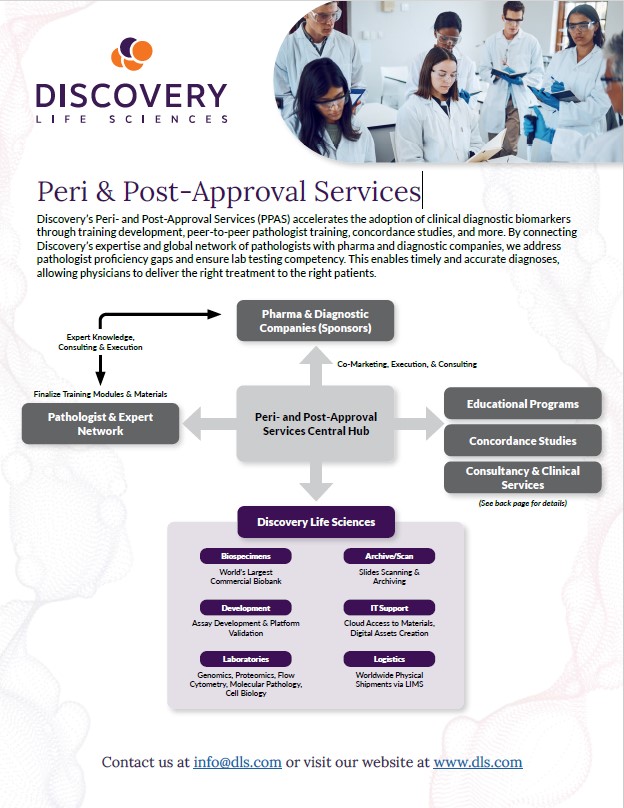
Discovery’s Peri- and Post-Approval Services (PPAS) accelerates the adoption of clinical diagnostic biomarkers through training development, peer-to-peer pathologist training, concordance studies, and more. By connecting Discovery’s expertise and global network of pathologists with pharma and diagnostic companies, we address pathologist proficiency gaps and ensure lab testing competency. This enables timely and accurate diagnoses, allowing physicians to deliver the right treatment to the right patients.


