
Over the last decade, advances in immuno-oncology have created entirely new therapeutic options for previously untreatable or underserved cancer indications, increasing both life expectancy and quality of life for patients with previously untreatable cancers. As we push back the limits of what can be achieved in the treatment of cancer with these new therapeutics, an ever deeper and more profound understanding of the underlying tumor biology is required, both to develop new treatments, and to target the existing ones to the patients who benefit.
New techniques in spatial biology can provide insights into the tumor microenvironment (TME) with unprecedented levels of detail and the cell-to-cell interactions amongst and between cell types. It is often this spatial arrangement that defines the TME, and that endows individual tumors with strengths and weaknesses that render them vulnerable to certain therapeutics but immune to others. Combined with the advancement in imaging technologies, a new approach in examining complex tumor biology has emerged, making possible insights and discoveries that would have previously been obscured.
This is particularly important for immuno-oncology where not just the presence, but the spatial distribution of biomarkers within the complex three-dimensional space of the TME is proving to be critical in determining the likely efficacy of a therapeutic. First demonstrated by Tumeh et al [1] using quantitative IHC and mIF to investigate patient response to pembrolizumab in advanced melanoma, researchers found that patient response was determined not simply by the presence or absence of the target PD-L1, but by the co-localization of PD-L1 in close proximity to PD-1 expressing CD8+ T-cells along the tumor’s invasive margin. A number of other studies have since reinforced the importance of the spatial arrangement and localization of biomarkers within the tumor for predicting responsiveness to therapy across other cancer indications including metastatic melanoma [2] and non-small cell lung cancer [3].
Spatial analysis of the tumor micro-environment with multiplex immunofluorescence
Multiplex immunofluorescence (mIF) provides detailed spatial imaging of tissue samples including formalin-fixed paraffin-embedded (FFPE) tumor tissue typical in clinical trials. Utilizing the simultaneous detection of multiple biomarkers on a single tissue section and the visualization of the spatial distribution of proteins within a tissue sample to enable deep tissue phenotyping, mIF is a potent tool for the detailed characterization of the TME [4] helping drug developers to better understand the response to immuno-oncology drugs, accelerate the development of more sophisticated prognostic biomarkers, and develop more accurate companion diagnostic tests required to better align treatment regimens to individual patients. [5]
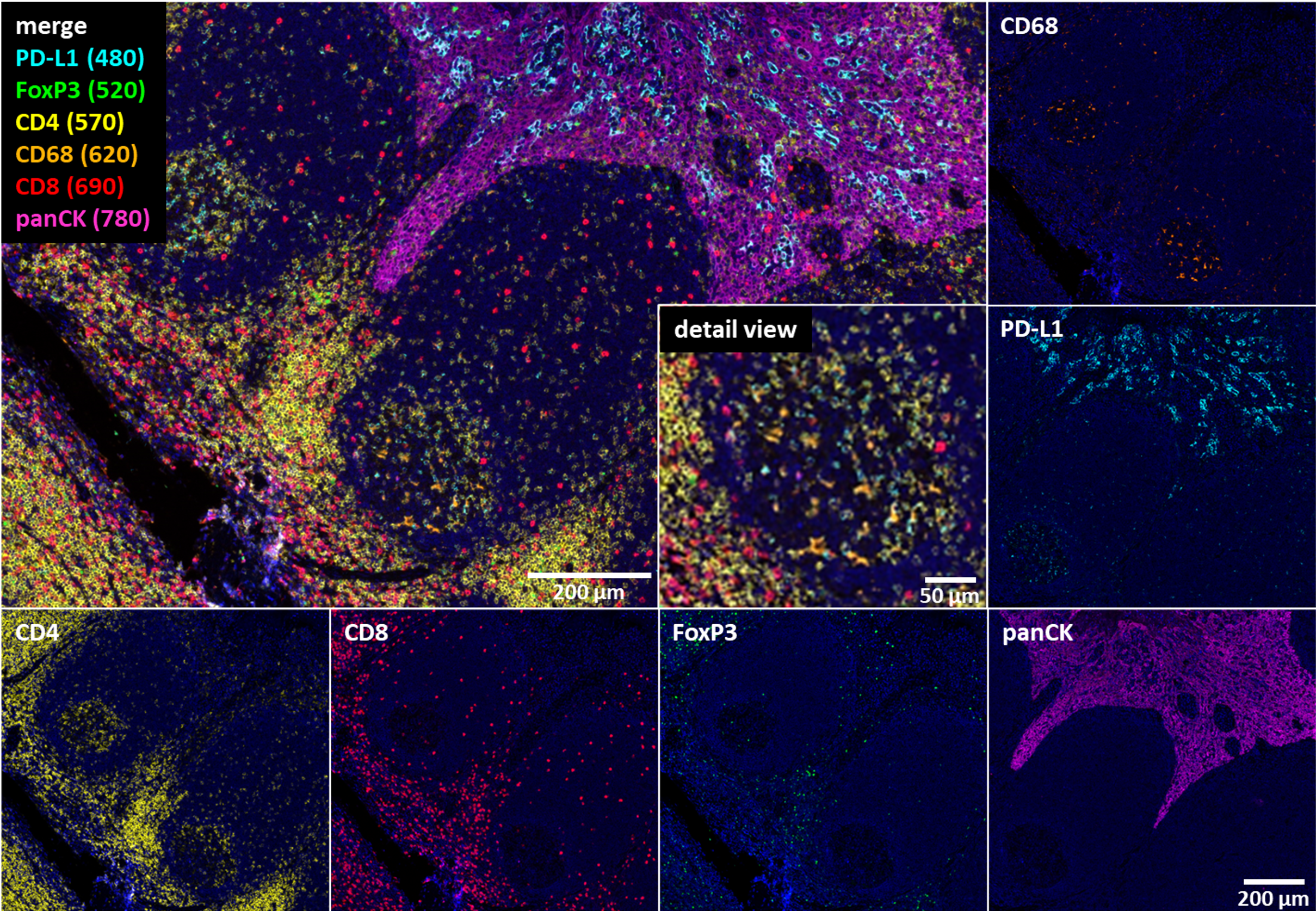
Figure. 1. Multiplex immunofluorescence using 6-fluorophores creates a detailed image of the special distribution of cells and proteins in normal tonsil tissue, revealing the relative positions of different cell types and ligands distributed throughout the sample.
While the established tools of immunohistochemistry (IHC) are still the gold standard in prevalence screening providing highly specific and sensitive assessments of tissue samples, immuno profiling with IHC has its boundaries. IHC is limited by the number of proteins it can characterize per slide and its compatibility with computer algorithms due to fluctuating image quality. IHC also requires a significant amount of tissue because only one or two biomarkers can be used per slide. This presents a problem, especially in retrospective studies that are reliant upon existing, often limited sets of clinical samples, and which constrains the number of studies that can be performed and the amount of information that can be gleaned from available samples.
mIF addresses these constraints, producing highly detailed images that provide spatial information on the relationships and interactions between different cell types within the tumor. mIF imaging displays clinically relevant details such as cellular co-expression, cellular spatial relationships, tissue heterogeneity, and expression of low abundance molecules and the distribution of these within the three-dimensional tissue structure in a manner not possible with IHC. mIF not only detects the presence of a biomarker in a tissue sample but enables researchers to determine the relevance and impact of that biomarker based on its localization and connection to other cell types.
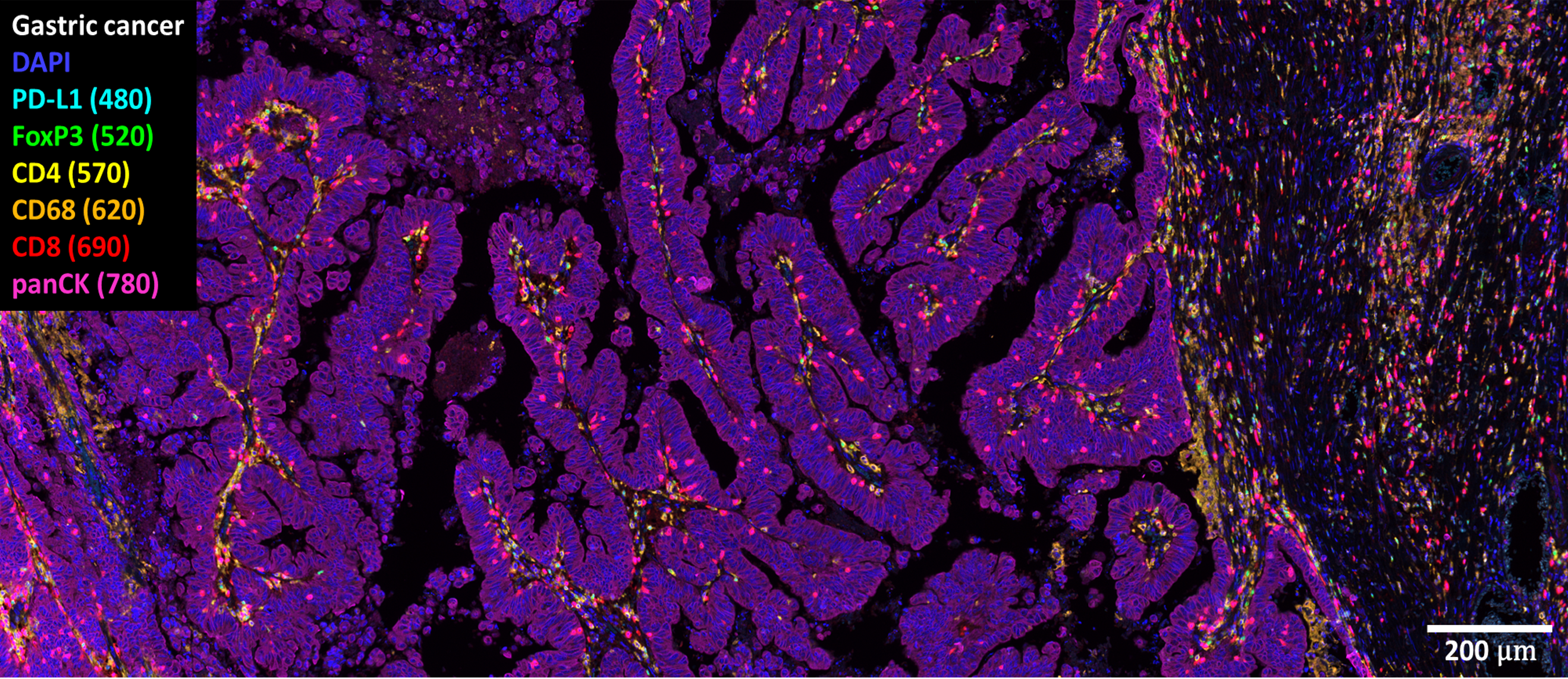
Figure. 2. Multiplex immunofluorescence analysis on gastric cancer tissue.
Discovery Life Sciences’ robust, validated mIF assay development process
Building mIF assays is a more complex, multistep process than the development of traditional IHC staining assays, requiring the optimization of each individual immune fluorescent (IF) marker in turn, followed by optimization and validation of the multiplex assay. Discovery Life Sciences (Discovery) has a robust and reliable mIF assay development process that draws heavily on its strong expertise in histopathology, as well as experience in virtual microscopy powered by artificial intelligence and machine learning. For assays that are used in decision-making during clinical trials it is also important that they are validated following good clinical laboratory practices (GCLP). Discovery has developed a state-of-the-art, pathologist-driven, and GCLP-compliant procedure for the rapid development and validation of custom multiplex immunofluorescence assays. These assays and their associated AI-driven digital image analysis algorithms are also developed in CAP-accredited, CLIA-certified laboratories by our experts.
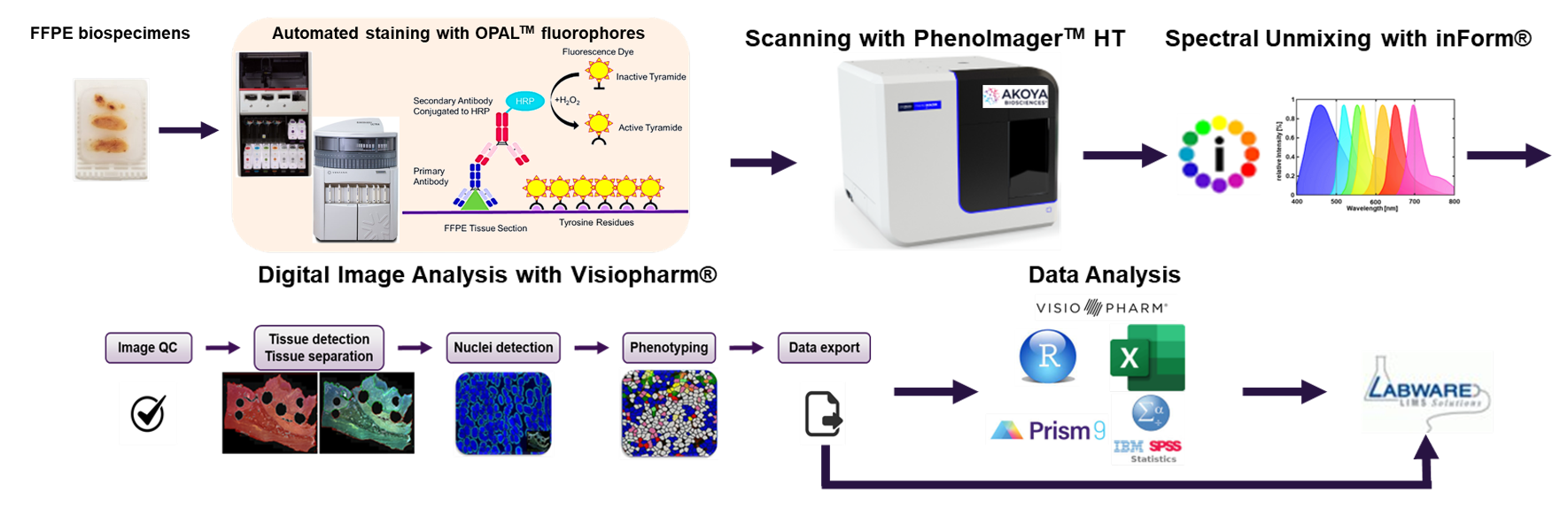
Figure. 3. Discovery multispectral imaging of complex tissue samples using multiplex immunofluorescence
Discovery’s protocol can generate multispectral assays with up to 6 different IF markers for full tissue analysis, and up to 8 different markers for focused regions of interest. Following each staining step, epitope specificity and sensitivity are confirmed as well as antibody complex stripping efficiency for each target without impacting tissue morphology. Subjective human evaluation of slides is replaced by objective, automated imaging and quantitative, automated, calling using artificial intelligence and deep learning algorithms. Also, one key to the rapid development of new mIF assays is ready access to relevant diseased and control tissue samples. Discovery maintains the world’s largest commercial biospecimen inventory and procurement network which enables the rapid acquisition of clinical samples and reduces development time for new assays.
A promising approach for cancer immunophenotyping in clinical trials
As we enter a new chapter in immuno-oncology, deeper and more profound understanding of the tumor biology is needed. The power of mIF to reveal the inner workings of the tumor microenvironment and to dissect the delicate and complex biological interactions between different cell types is truly remarkable, but the development of these new assays is a complex multi-disciplinary process requiring expertise across a variety of different fields as well as the ability to draw on rare diseased tissue samples. Discovery has over 20 years of experience in pathology and brings all the necessary disciplines under one roof for rapid development and deployment of robust custom mIF assays. Moreover, the mIF findings can be orthogonally assessed using Discovery’s wide range of multi-omic biomarker services in genomics, proteomics, and cell biology. By partnering with a reliable biomarker expert early in the drug development process, deeper insights can be uncovered without over-consuming precious clinical samples and more specific patient populations can be identified for clinical trials improving the program success rate.
References
- Tumeh et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature. 515 (2014) Vol. 7528 pp. 568-571. https://doi.org/10.1038/nature13954
- Wong et al. Quantitative Assessment of Tumor-Infiltrating Lymphocytes and Immunotherapy Outcome in Metastatic Melanoma. Cancer Res. 78 (13). https://doi.org/10.1158/1538-7445.Am2018-3638
- Mazzaschi et al. Low PD-1 Expression in Cytotoxic CD8+ Tumor-Infiltrating Lymphocytes Confers an Immune-Privileged Tissue Microenvironment in NSCLC with a Prognostic and Predictive Value. Clin Cancer Res. 2018 Jan 15;24(2):407-419. https://doi.org/10.1158/1078-0432.CCR-17-2156
- Tsujikawa et al. Quantitative multiplex immunohistochemistry reveals myeloid-inflamed tumor-immune complexity associated with poor prognosis. Cell reports. Vol. 19 Issue 1. 4 April 2017. Pages 203-217. https://doi.org/10.1016/j.celrep.2017.03.037
- Binnewies et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine. 24 (2018), pp. 541-550 https://doi.org/10.1038/s41591-018-0014-x


