Scalable Workflow for Single Nuclei Genomics and In Situ Hybridization
Discovery’s Annual Year-End Sales Event is Here! Explore Exclusive Deals Available Now.
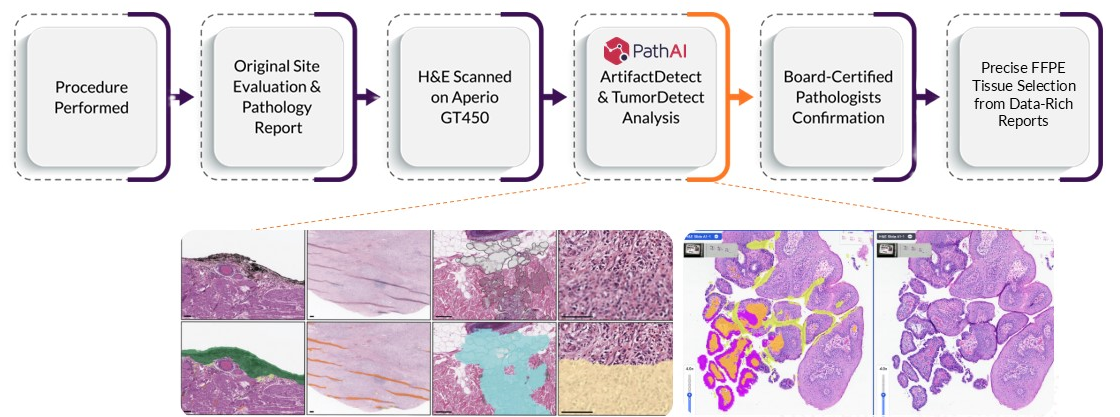
White Paper - Companion Diagnostic Development: An Overview
NEW App Note: Optimized siRNA and mRNA Delivery in Primary Mouse Hepatocytes for Gene Therapy
Scalable Workflow for Single Nuclei Genomics and In Situ Hybridization
Discovery’s Annual Year-End Sales Event is Here! Explore Exclusive Deals Available Now.
White Paper - Companion Diagnostic Development: An Overview
NEW App Note: Optimized siRNA and mRNA Delivery in Primary Mouse Hepatocytes for Gene Therapy