The genomics landscape is constantly evolving as more genetic variants and epigenetic patterns are identified as biomarkers of disease progression, remission, or drug response. Precision medicine, a new, more personalized way to diagnose and treat patients based on genetic biomarkers, is driven by these emerging insights from advanced technology, analysis, and bioinformatics. In particular, there are many oncology indications with genomic variants that affect how the patient responds; these therapies can be designed specifically for these targets instead of a broader approach to cancer treatment like surgery, radiation, and chemotherapy. At Discovery, we have solutions that allow for tumor characterization as well as many other tissues/fluids across disease types to advance precision medicine with experts to design and validate clinical trial biomarkers every step of the way.
Discovery is uniquely positioned to help scientists overcome challenges in precision oncology with large-scale, innovative sequencing services. “Our expertise is centered on high-throughput sequencing, workflow optimization, and developing cutting-edge analytical pipelines in support of global, large-scale clinical trial programs. We routinely help researchers move beyond challenges to advance biomarker programs, develop and validate new targeted therapies, immunotherapeutics, liquid biopsy diagnostics and companion diagnostics.
Dr. Shawn Levy, PhD
Chief Scientific Officer
Discovery Life Sciences
3 Challenges Biopharma Companies Face in Precision Oncology Programs
1. Integrating evolving and complex genomic technologies into development programs:
In the field of genomics, the 21st century brought rapid discovery, transformative technological development and significantly decreased costs. The field has graduated from the human genome project, which involved international resources, took more than a decade to complete, and cost billions of dollars, to sequencing individual genomes quickly at a fraction of the cost. This explosion of technological advances creates a paradox for researchers leading precision oncology programs. On one hand, innovative technologies provide new, revolutionary ways to gain genomic insights that can create new paradigms in patient treatment. On the other hand, more information introduces myriad questions for developers to carefully navigate. Which technologies are best to solve complex, multi-faceted questions during drug and diagnostic development? How should the data from these technologies be interpreted and validated? Are there improved sample preparation, processing or workflow advances that can help save time, money, and unlock insights from samples previously thought impossible?
2. Embracing and Utilizing Multi-omics:
There is a substantial array of expanding requirements for multi-layered approaches across each individual for biomarker characterization in drug development and diagnostics. Use of genetics, transcriptomics, epigenomics, proteomics, metabolomics, and digital pathology to analyze tumors, adjacent tissues, peripheral blood, and other body fluids alongside patient history and clinical outcome data are needed for drug and diagnostic developers to construct a comprehensive profile of the patient’s condition and identify targets for treatment or stratification.
3. Leveraging Data to Stratify Patients in Context:
In addition, it’s challenging to accurately stratify patients to increase efficacy and safety of novel therapeutics. While larger panels and multi-omic testing can increase the likelihood of biomarker discovery, they also create unactionable noise. To interpret increasingly complex data sets, strategic analytical and bioinformatics approaches are required to ensure quality control, accurate data analysis, biomarker discovery, and validation. Compounding data issues is the need to ensure the data is produced in context, i.e. study cohorts must comprise a diverse range of samples from patients that mirror intended use populations prior to clinical trials, so developers can manage appropriate applications of genomic data and their impact on decision making during development.
How Discovery Helps Overcome These Challenges
To overcome these challenges, Discovery provides unmatched access to highly characterized samples, innovative, optimized services on cutting-edge multi-omic platforms, expert assay establishment and validation, and intricate data analysis / bioinformatics* to ensure accuracy, save time, and eliminate wasted resources.
EVALUATE, VALIDATE AND SCALE NOVEL SEQUENCING TECHNOLOGIES TO ACCELERATE PRECISION ONCOLOGY PROGRAMS
Discovery’s sequencing and bioinformatics service lab, HudsonAlpha Discovery®, offers the latest genomic innovations delivered at speed and scale—proprietary workflows, advanced sequencing platforms, and the Discovery Life Sciences commitment to service. Our experts work with clients consultatively to better understand their needs and provide recommendations on experimental design, technology selection and application, custom workflow optimizations, etc.
We have been chosen as the sequencing engine behind population-wide single cell, short- and long-read sequencing studies due to our ability to rapidly evaluate, validate and adopt novel sequencing technologies, so we can provide reliable data on emerging and well established genomic technologies at any scale for discovery and clinical trial applications. We will continue working directly with genomic technology companies like Illumina, PacBio and 10X to help them advance their technologies’ applications while leveraging those same technologies to build large-scale services with optimized sample handling and customized workflows that help drug and diagnostic developers more effectively navigate and accelerate their precision oncology programs.
Dr. Shawn Levy, PhD
Chief Scientific Officer
Discovery Life Sciences
NovaSeq 6000
Quantity = 10
NovaSeq 550
Quantity = 2
MiSeq
Quantity = 2
iSeq 100
Quantity = 1
Sequel IIe
Quantity = 10
PromethION
Quantity = 1
Chromium Controller
Quantity = 1
Chromium Connect Automated Controller
Quantity = 1
nCounter Flex
Quantity = 1
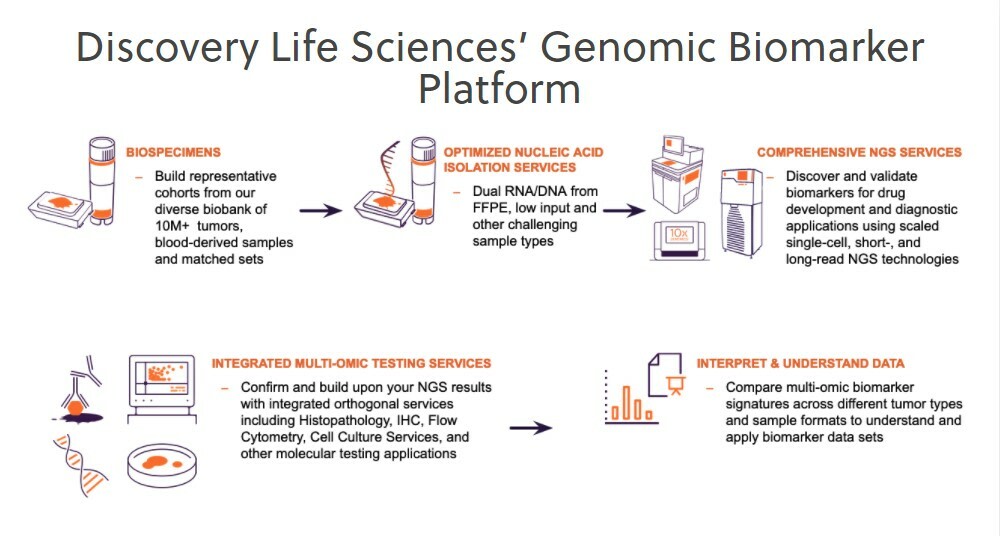
Discovery’s Integrated Multi-omic Services
Our global biomarker service laboratories routinely manage hundreds of global studies and expertly test thousands of biospecimens simultaneously using NGS, IHC, flow cytometry and other technologies. We’ve published or been cited in more than 500 peer-reviewed publications across all these disciplines and have successfully applied our services in every stage of development.

Review our peer-reviewed publications
SEE HOW YOUR COLLEAGUES ARE LEVERAGING OUR SOLUTIONS-
Realizing Precision Oncology
Cancer tumors vary widely and are extremely diverse in their growth and mutation. Targeted therapies are the future of the genomics landscape. In Immuno-oncology, specific biomarkers such as Tumor Mutational Burden (TMB) and immune checkpoint protein target expression (e.g., PD-1 and PD-L1), provide broader biomarker signatures and opportunities for alternative means via combination and/or adjuvant therapies. But to realize and expand these advances, developers must have reliable data and methods to interpret data properly.
Don Skifter, PhD., MBA is Discovery’s SVP of Genomics whose expertise is focused on how clients can apply genomic technologies and bioinformatics analyses to advance researchers’ understanding of disease and development of diagnostics, drugs and therapies that improve patient care.
Biomarker programs have evolved to look across a variety of types of biomarkers including the stacking of molecular, proteomic, imaging, and other patient-specific traits on top of one another to evaluate more complex biomarker signatures. At Discovery, we offer these multi-layered services as a single strategic partner, preserving precious clinical trial tissue and fluid samples for multi-omic analyses. Taken together, our breadth of high-quality lab service and biospecimen procurement capabilities combined with our collaborative “Science at Your Service™” approach help drive novel insights for new drug targets and to characterize the predictive and pharmacodynamic biomarkers that enable patient stratification and individualized drug response.
Don Skifter, PhD., MBA
Discovery’s SVP of Genomics
Discovery Life Sciences
Correctly identify, develop and properly validate biomarker assays
In clinical trials, one challenge many pharma companies have is they’re unsure which biomarker to use when evaluating patient samples. However, based on the expression data, companies can design their own clinical studies with Discovery to get a better understanding of biomarker expression, especially in the cases of annotated samples and mutation status, etc. It’s important to have a variety of technologies and capabilities that can be applied, such as IHC, digital pathology, NGS, molecular and flow cytometry capabilities.
SEE OUR BIOSPECIMEN SOLUTIONS AND MULTIOMIC SERVICES IN ACTION:
A Pembrolizumab Case Study
References
https://www.ajmc.com/view/how-advances-in-precision-oncology-new-diagnostic-and-treatment-frontier
*For Research Use Only. Not Intended for use in Diagnostic Procedures.




